What role could convalescent sera play in the COVID-19 pandemic?
June 2, 2020
Dr. Tyler Bold, Assistant Professor, DOM, and Dean’s Scholar in Microbiology, Immunology and Infectious Diseases, replies:
Sera from patients who have recovered from SARS-CoV-2 infection (convalescent sera) could be used immediately to provide protective immunity and treat the severe and life-threatening pneumonia the virus causes, while the urgent quest continues to develop vaccines and treatments of proven safety and efficacy.
Let’s start with the difference between conventional vaccines and convalescent sera for prevention. Active immunization, the basis for most modern vaccines, involves exposing a healthy individual to antigenic components of a pathogen in order to elicit durable immune protection (termed “immunologic memory”) against subsequent infection with the same pathogen. In contrast, for passive immunization against SARS-CoV-2, convalescent sera with antibodies that neutralize the virus would be transferred to an exposed individual or COVID-19 patient to prevent or treat infection (Figure 1).
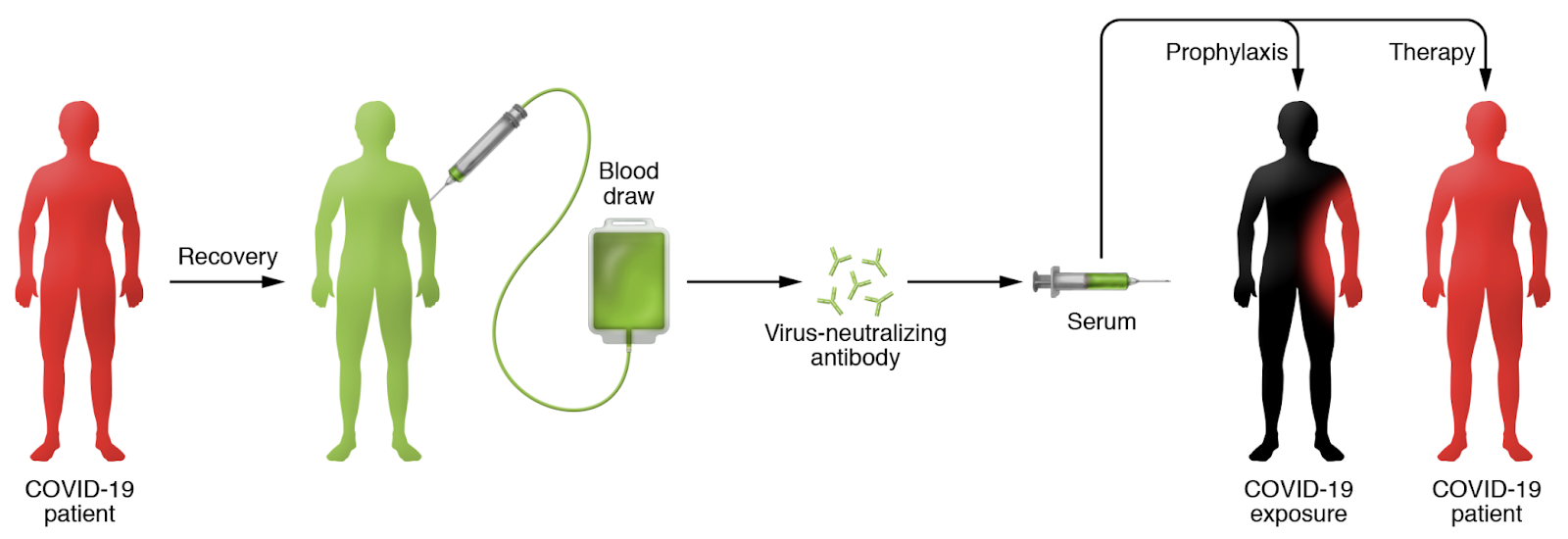
Figure 1 excerpted from Casadevall and Pirofski, JCI 2020
The concept of passive immunization for protection against and treatment of infectious diseases has a long history going back to the 1890s for treatment of diphtheria and thereafter for many bacterial infections. The advent of effective antibiotics largely supplanted passive immunization, but there remain several well-established, highly effective examples of passive immunization in use today: hepatitis B immune globulin, administered to HBV-infected pregnant women to prevent perinatal transmission; and human rabies immune globulin, administered after a potential rabies exposure. Passive immunization has also been used experimentally in the context of several outbreaks of viral infections that lack effective treatments, including Ebola, SARS, MERS, H1N1 influenza, and notably, during the 1918 influenza pandemic.
For the COVID-19 pandemic there is thus a strong rationale to support this approach. But, what data do we have to prove that passive immunization with convalescent sera works, and in what context would this approach be safest and most effective? Since many individuals in the world have now been infected with SARS-CoV-2 and have recovered from COVID-19, there now exists a reasonably large pool of individuals who may potentially carry protective antibodies against this virus. In the US, there are currently 24 actively recruiting clinical trials of COVID-19 Convalescent Plasma (CCP), in which plasma is obtained from recovered individuals (donors) and transfused into recipients who are either already infected or are at risk of becoming infected with SARS-CoV-2 (Figure 1). A New York study that compared COVID-19 outcomes in 39 subjects who received CCP to a matched group of controls who did not, was published on MedRxiv on May 22 (Liu et al). The CCP recipients had a slight improvement in oxygen requirement and better survival just in the less severe subgroup of non-intubated patients who were likely at an earlier stage of lung infection.
Is CCP available to M-Health/Fairview patients?
CCP is currently available to M-Health/Fairview patients by way of the Mayo Clinic Expanded Access Program (EAP) IND. Claudia Cohn, the director of the Blood Bank Laboratory, is administering this program. CCP is currently available to hospitalized adults with laboratory-confirmed diagnosis of SARS-CoV-2 infection, and either severe or life-threatening COVID-19, or a high risk of progression to severe or life-threatening disease. The dose is two units of CCP given within 12 hours. To conserve inventory, CCP has initially only been given to patients outside the ICU. This is consistent with a conceptual understanding that treatment earlier in disease is more likely to be effective, supported by the findings of Liu et al. Most of our CCP units have come directly from New York City because Memorial Blood Center is affiliated with New York Blood Center. We are trying to improve donations by developing a system of directly contacting our discharged COVID patients to encourage them to donate. As inventory improves there is a plan to release CCP units to any patient who is eligible, based on their clinical team’s decision.
As of May 22nd, 53 M Health Fairview patients have received CCP: 17/53 (32%) have been discharged; 7/53 (13%) are deceased. Of the 29 who remain hospitalized, 18 have required mechanical ventilation, and 5 of these have been extubated. CCP therapy appears to be safe, so far, with no cases meeting criteria for transfusion associated lung injury, and no clearly documented evidence of antibody dependent enhancement (ADE) of disease, a theoretical concern for passive antibody transfer.
Are there studies underway to determine if there are differences in the likely efficacy of individual CCP units?
While there is no current consensus on how to determine which CCP units are most likely to be beneficial to the recipient, one plausible hypothesis is that CCP units with the highest antibody titers or highest levels of antibodies that neutralize SARS-CoV-2 would be most beneficial. We have conducted in-house testing of the antibody binding activity of our donor CCP units in collaboration with Amy Karger and ARDL, using both the ARDL/Jenkins ELISA assay for SARS-CoV-2 Spike protein Receptor Binding Domain that binds to the cellular receptor to enable virus entry; and an FDA approved Roche clinical assay for Nucleocapsid protein. The results from the two assays were in excellent agreement, with a wide range in antibody levels (titers) ranging from 1:400 to 1:51,200, and notable for several CCP units non-reactive in both assays. Our current goal is to begin testing all CCP units for neutralization activity against live SARS-CoV-2 infection in vitro (in tissue cultures of susceptible cells). We will use the functional neutralization activity in individual CCP units to correlate with clinical outcomes such as survival and improvement in pulmonary function. If we find that the best outcomes correlate with Neutralizing antibody levels we can then select CCP units that are most likely to be beneficial to the recipient.
It seems very likely that some CCP donors and units will provide better protection against disease than others. As illustrated in a CBS 60 Minutes piece that aired May 31, 2020, some hospitals are developing strategies that incorporate these factors into CCP patient care decisions. Dr. David Perlin of the Center for Discovery and Innovation of Hackensack Meridian School of Medicine is quoted: “Donors are rated on a scale of one to four stars. The small number of recovered COVID-19 patients, who produce antibodies 10, 30, even 50 times more than others, are called ‘super donors.’” Developing a program at UMN/MHealth/Fairview to identify the characteristics of CCP that confer superior protection is an important component of ensuring the success of this therapeutic strategy for COVID-19.